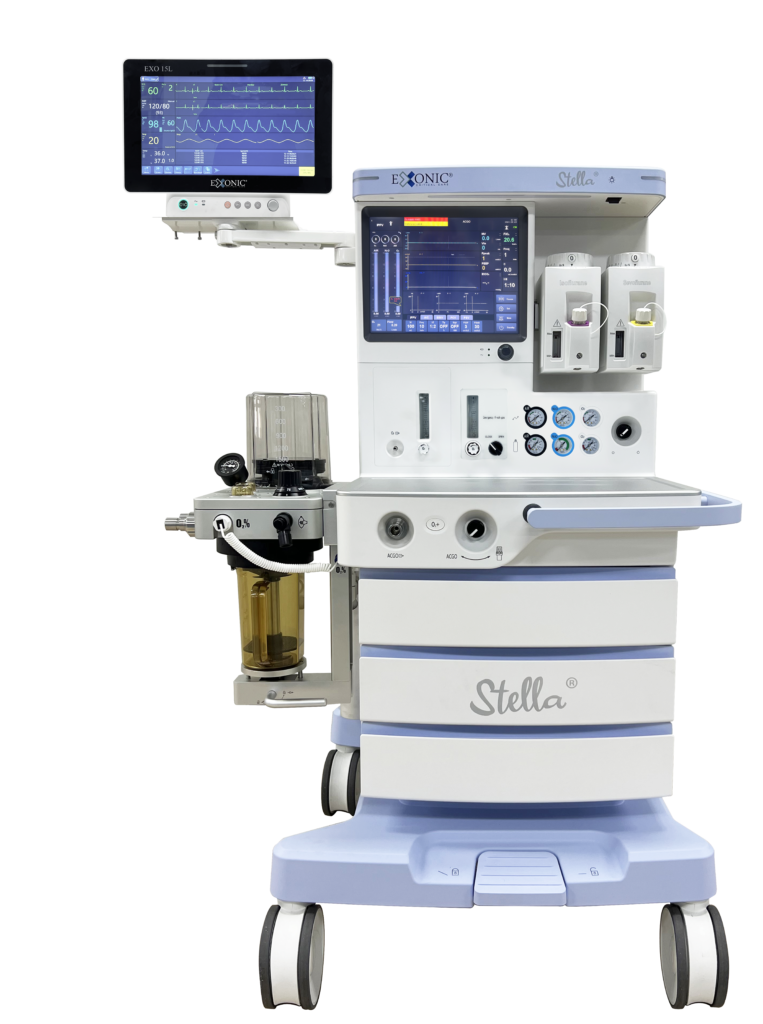
Empowering Clinicians, Enriching Lives
Exonic Designs and Manufactures Critical Care Equipments.
Exonic designs and manufactures critical care equipments, with a primary goal of meeting customer needs and being competitive in the medical field. We have dedicated over a decade to researching and innovating, specifically in the areas of anesthetic and intensive care, to produce and sell intelligent medical devices.
We strive for continuous development and innovation to provide
high-quality medical care services that promote healthy living. We
have established ourselves in cities throughout the USA and are committed to advancing medical life sciences in the Middle East with our forward-thinking vision, technical expertise, and scientific management.
The company has acquired ISO 9001/13485 international quality system certification, 14001 environmental management system certification, ROHSH environmental certification, GMP and CE certification. These certifications are a testament to our commitment to delivering high-quality products and services while also adhering to environmental and safety standards.
Moving forward, Exonic will harness the expertise of our research and technical teams and marketing advantages to integrate resources and seize strategic opportunities. We will continue to prioritize innovation as the basis for development and use our professionalism to build the Exonic brand. Our goal is to form an “overseas market-oriented” development pattern and become a leading medical device enterprise that integrates product research and development, production, and sales. With better services, Exonic is committed to enabling the public to enjoy a healthy future brought by our technology.

With a motto of technological innovations to serving hospitals
We have emerged as a leader in the medical device industry. We currently employ a workforce of 300 people, including more than 50 professional engineers. Our company has production plants that adhere to standardization and are equipped with advanced technical equipment and complete testing standards. With over two decades of experience in medical production, we have remained at the forefront of the industry’s process technology.
Exonic has established partnerships with several companies in various cities across the USA and other countries. The company has successfully executed several projects that are highly recognized within the industry. Currently, we are looking to extend our vision and open up new markets in Asia and Africa through strong agents.
Exonic considers the development of medical and health services
and is committed to introducing advanced production technologies and equipment from Germany, USA, and Japan to guarantee processing techniques. Our company mainly produces a wide range of critical care equipment, covering hospital, operating room, ICU/CCU, and emergency departments. Exonic has established a stringent quality management system, leveraging our commitment to first-class product quality and meeting customer needs. To achieve this, we have built a specialized quality inspection department that sources high-quality raw materials from qualified suppliers and enforces rigorous inspections and controls at every production stage.
During production, we follow strict disinfection procedures and ensure that people flow and logistics are separated. Every process is thoroughly inspected using precise instruments, guaranteeing a product pass rate of 100% in delivery inspections and market surveillance spot checks. This ensures that we maintain our reputation for delivering high-quality products that meet the needs of our customers. Exonic recognizes that customer satisfaction is essential to quality assurance and has therefore created a consulting service technical team to focus on “product service and expert consultation.” We have established offices, after-sales service, and technical support centers in several countries to provide consultation services on technical key points of medical devices and resolve any technical issues encountered by customers.
Empowering Healthcare Excellence with